BACTERIAL RESISTANCE
Treat your patients today, and for the future1–6
Throughout the world, antibiotic resistance is on the rise.7 You may limit its risk with ORACEA (doxycycline, USP)
40 mg* Capsules, a non antibiotic dose, which have been shown to be safe in a long-term study,† and learn just how quickly antibiotic resistance can develop with different doses of doxycycline2–5,8
*30 mg immediate release and 10 mg delayed release beads
†No evidence of bacterial resistance in a 9-month clinical study5
Sustained anti-inflammatory effect
AS POWERFUL AS a HIGH-DOSE DOXYCYCLINE
AT A NON-ANTIBIOTIC DOSE2,3
- Sustained anti-inflammatory effects2,3
- Low plasma variability over 24 hours2,3
- Does not cross the antimicrobial threshold1-3,5,9,10
Mean plasma concentrations over 24 hours2,3
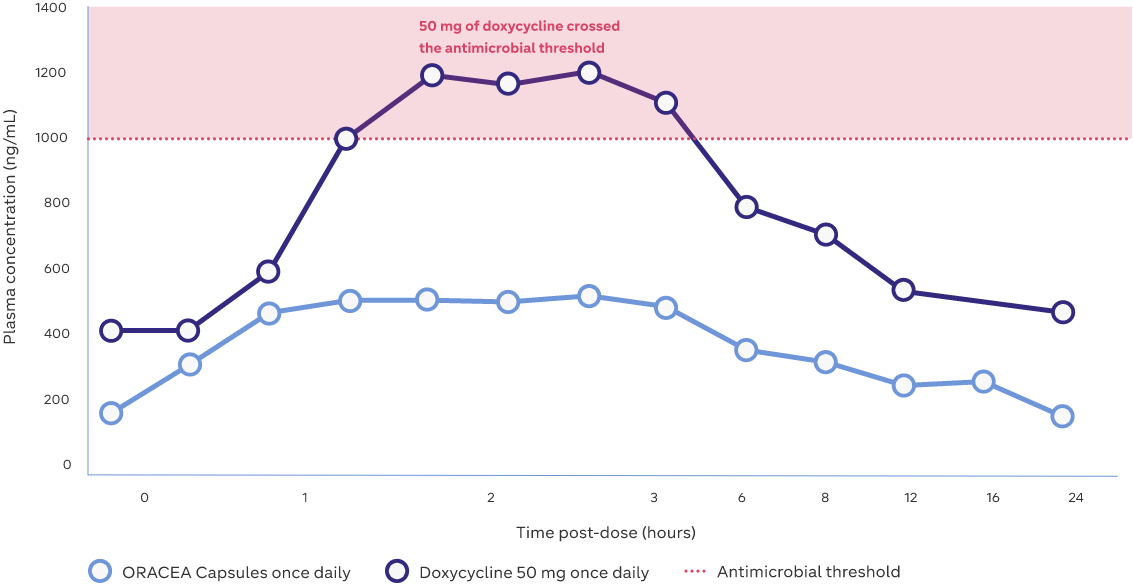
Study Design: Sixteen healthy subjects in the ORACEA Capsules arm measured at 7 days; mean weight 75 kg. Values plotted on the graph indicate mean plasma concentrations over 24 hours2,3
Adapted from: Fowler JF. Anti-inflammatory dose doxycycline for the treatment of rosacea. Expert Rev Dermatol. 2007;2(5):523–531
Antibiotic resistance
Doxycycline 100 mg caused antibiotic resistance in 7 days3,8
- Subjects discontinued doxycycline 100 mg after 14 days8
- But antibiotic resistance remained elevated for the 2 weeks following8
Microbial resistance following doxycycline (100 mg) once daily8
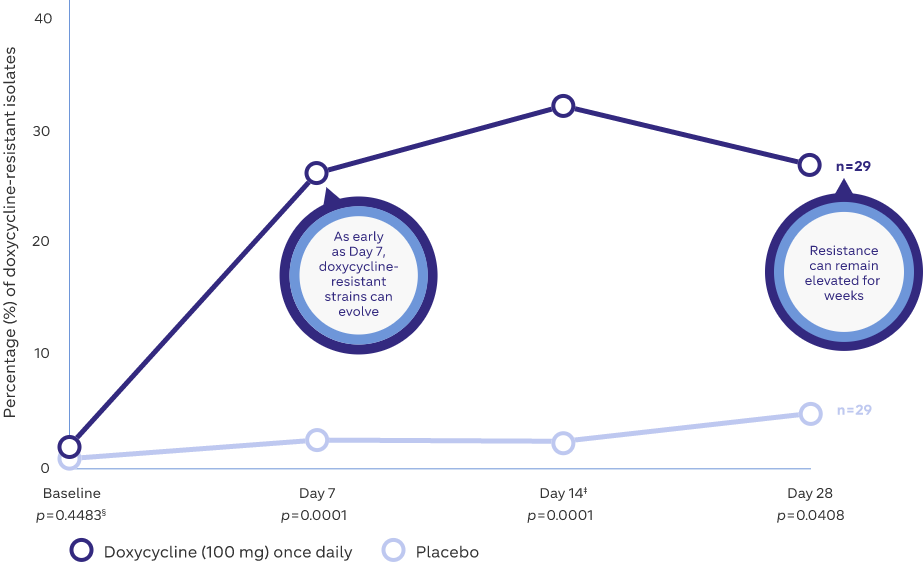
Study Design: A prospective, placebo-controlled, randomized, double-blind trial where 29 subjects (healthy volunteers) either received 100 mg doxycycline or placebo once-daily for 14 days8
Adapted from: Walker C and Bradshaw M. The effect of oral doxycycline 100 mg once-daily for 14 days on the nasopharyngeal flora of healthy volunteers: a preliminary analysis. Presented at:
Fall Clinical Dermatology; October 18–21, 2007; Las Vegas, NV.
‡Subjects discontinued doxycycline 100 mg on Day 14
§p values compared with placebo, by Mann Whitney U test
![]()
Oracea capsules
See how the unique formulation of
ORACEA Capsules helps treat your patients with
inflammatory lesions of rosacea
STAY CONNECTED
Sign up and
stay up-to-date
For the latest news, research, and insights into papulopustular rosacea, subscribe now! You’ll also receive patient resources and toolkits, helping you treat your patients throughout their rosacea journey
Important Safety Information
Indication: ORACEA® (doxycycline) 40 mg* capsules are indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. ORACEA does not lessen the facial redness caused by rosacea. Adverse Events: In controlled clinical studies, the most commonly reported adverse events (>2%) in subjects treated with ORACEA were nasopharyngitis, diarrhea, hypertension and sinusitis. Warnings/Precautions: ORACEA should not be used to treat or prevent infections. ORACEA should not be taken by patients who have a known hypersensitivity to doxycycline or other tetracyclines. ORACEA should not be taken during pregnancy, by nursing mothers, or during tooth development (up to the age of 8 years) and may cause reversible inhibition of bone growth. If Clostridium difficile associated diarrhea (CDAD) occurs, may need to discontinue ORACEA. Although photosensitivity was not observed in clinical trials, ORACEA patients should minimize or avoid exposure to natural or artificial sunlight. The efficacy of ORACEA treatment beyond 16 weeks and safety beyond 9 months have not been established.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
*30 mg immediate release and 10 mg delayed release beads
REFERENCES
1. Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycl ine, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. 2. Fowler JF. Anti-inflammatory dose doxycycline for the treatment of rosacea. Expert Rev Dermatol. 2007;2(5):523–531. 3. Baldwin HE. Diagnosis and treatment of rosacea: state of the art. J Drugs Dermatol. 2012;11(6):725–730. 4. Data on file. ORACEA PK Profile. Galderma Laboratories, L.P. 5. Preshaw PM, et al. Modified-release sub-antimicrobial dose doxycycline enhances scaling and root planning in subjects with periodontal disease. J Periodontol. 2008;79(3):440–452. 6. Etchegaray JP, Wagner N, Shah MS, Difalco RJ, inventors; Galderma S.A., Cerovene, Inc. assignees. Doxycycline formulations, and methods of treating rosacea. US Patent 8,652,516 Bl. February 18, 2014. 7. Ventola CL. The antibiotic crisis. Part 1: Causes and threats. P&T 2015;40:277–283. 8. Walker C and Bradshaw M. The effect of oral doxycycline 100 mg once-daily for 14 days on the nasopharyngeal flora of healthy volunteers: a preliminary analysis. Presented at: Fall Clinical Dermatology; October 18–21, 2007; Las Vegas, NV. 9. Bhatia N. ORACEA 40 mg capsules for papulopustular rosacea. The Dermatologist. 2013;6(21s):1-4. Available at: https://www.hmpgloballearningnetwork.com/site/thederm/site/cathlab/event/oracea-40-mg-capsules-papulopustular-rosacea-1. Last accessed: September 2021. 10. Wise RD. Submicrobial doxycycline and rosacea. Compr Ther. 2007;33(2):78–81



