SAFETY & TOLERABILITY
Rosacea treatment without compromise:
simple, safe and effective1–3
Find out how ORACEA® (doxycycline, USP) 40 mg* Capsules delivers a strong safety profile for the long term†, as well as information on how the non-antibiotic dose of ORACEA Capsules provides real-world benefits for your papulopustular rosacea patients1–4
Favorable tolerability to help patients stay on track1,2
Pivotal Data
A ONCE-DAILY DOSE YOUR PATIENTS CAN TOLERATE1,2
- Adverse events similar to placebo1
- No reported photosensitivity in controlled clinical trials vs placebo1
- No reported vaginal candidiasis1
In 2 pivotal clinical studies, the most common treatment-related adverse
events (>2%) for each drug were:1
| Most common adverse events (%) | ORACEA Capsules n=269 |
Placebo n=268 |
|---|---|---|
| Nasopharyngitis | 4.8% | 3.3% |
| Diarrhea | 4.4% | 2.6% |
| Hypertension | 2.9% | 0.7% |
| Sinusitis | 2.6% | 0.7% |
| Elevated AST‡ | 2.2% | 0.7% |
Study Design: The safety and efficacy of ORACEA Capsules in the treatment of inflammatory lesions (papules and pustules) of rosacea was evaluated in two randomized, placebo-controlled, multi centered, double-blind, 16-week Phase 3 trials involving 537 subjects (total of 269 subjects on ORACEA Capsules from the two trials) with rosacea (10 to 40 papules and pustules and two or fewer nodules)1
*30 mg immediate release and 10 mg delayed release beads
†No evidence of bacterial resistance in a 9-month clinical study3
‡AST, aspartate aminotransferase
DOXYCYCLINE DATA
SUPERIOR GI TOLERABILITY VS DOXYCYCLINE 100 MG2
- Fewer GI side effects vs doxycycline 100 mg2
- No nausea, diarrhea or vomiting experienced with ORACEA Capsules2
In a head-to-head clinical study, the most common treatment-related adverse
events (>2%) for each drug were:2
| Adverse events (%) | ORACEA Capsules n=44 |
Doxycycline (100 mg) n=47 |
|---|---|---|
| Nausea | 0% | 17% |
| Headache | 4.5% | 6.4% |
| Influenza | 0% | 6.4% |
| Nasopharyngitis | 6.8% | 4.3% |
| Urticaria | 2.3% | 4.3% |
| Diarrhea | 0% | 4.3% |
| Esophageal pain | 0% | 4.3% |
| Vomiting | 0% | 4.3% |
| Abdominal pain | 0% | 2% |
| Abdominal pain upper | 0% | 2% |
Study Design: A randomized, multicenter, outpatient, double-blind, active-controlled, non-inferiority trial of 91 rosacea subjects (≥18 years of age) for 16 weeks. Patients were prospectively randomized to receive daily doses of either ORACEA Capsules or doxycycline (100 mg), each in combination with metronidazole cream, 1%2
HUMANA DATA
REAL-WORLD BENEFITS OF A SUB-ANTIMICROBIAL DOSE4
- In a retrospective analysis of a Humana database for the associated prevalence of GIDs in over 77,000 rosacea
patients treated with a sub-antimicrobial dose (SD) of 40 mg elicited fewer overall GID events vs conventional
doxycycline (CD§) of 50 mg or 100 mg4 - Reduced incidence of gastrointestinal disease (GID)4
50/100 mg doxycycline was
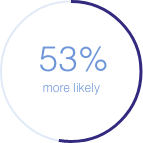
to be associated with GID vs
no doxycycline use**
1.53 (1.48-1.58); p<0.0001
However, SD doxycycline†† had a

GID event rate to
no doxycycline
1.05 (0.98-1.12); p=0.16
Study Design: A cross-sectional study of individuals with a history of rosacea between 2007-2015 (n=77,177) was conducted to examine the association between doxycycline treatments (40, 50, 100 mg, and no doxycycline) and 10 gastrointestinal diseases4
Adapted from: Lim HG, et al. Prevalence of gastrointestinal comorbidities in rosacea: Comparison of subantimicrobial, modified release doxycycline versus conventional release doxycycline. Journal of the American Academy of Dermatology. 2018;78(2):417–419
§Conventional dose doxycycline defined as the rosacea population (aged 30-64) with standard dose (50 or 100 mg), conventional dose doxycycline intake and without sub-antimicrobial dose intake (n=21,790)
**Rosacea population aged 30-64 without CD-doxycycline intake and without SD-doxycycline intake (n=50,492)
††Sub-antimicrobial dose doxycycline (SD) defined as the rosacea populated aged 30-64 with SD-doxycycline intake (n=4,835)
STAY CONNECTED
Sign up and
stay up-to-date
For the latest news, research, and insights into papulopustular rosacea, subscribe now! You’ll also receive patient resources and toolkits, helping you treat your patients throughout their rosacea journey
Important Safety Information
Indication: ORACEA® (doxycycline) 40 mg* capsules are indicated for the treatment of only inflammatory lesions (papules and pustules) of rosacea in adult patients. ORACEA does not lessen the facial redness caused by rosacea. Adverse Events: In controlled clinical studies, the most commonly reported adverse events (>2%) in subjects treated with ORACEA were nasopharyngitis, diarrhea, hypertension and sinusitis. Warnings/Precautions: ORACEA should not be used to treat or prevent infections. ORACEA should not be taken by patients who have a known hypersensitivity to doxycycline or other tetracyclines. ORACEA should not be taken during pregnancy, by nursing mothers, or during tooth development (up to the age of 8 years) and may cause reversible inhibition of bone growth. If Clostridium difficile associated diarrhea (CDAD) occurs, may need to discontinue ORACEA. Although photosensitivity was not observed in clinical trials, ORACEA patients should minimize or avoid exposure to natural or artificial sunlight. The efficacy of ORACEA treatment beyond 16 weeks and safety beyond 9 months have not been established.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
*30 mg immediate release and 10 mg delayed release beads
REFERENCES
1. Del Rosso JQ, et al. Two randomized phase Ill clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. 2. Del Rosso JQ, et al. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7(8):573–576. 3. Preshaw PM, et al. Modified-release sub-antimicrobial dose doxycycline enhances scaling and root planning in subjects with periodontal disease. J Periodontol. 2008;79(3):440–452 4. Lim, HG, et al. Prevalence of gastrointestinal comorbidities in rosacea: Comparison of sub-antimicrobial, modified release doxycycline versus conventional release doxycycline. J Am Acad Dermatol. 2018; 78(2): 417-419






